Introduction
As we all know the need for energy is been increasing day by day in our world. As new appliances are bought by us in our homes, our daily energy expense has been increasing resulting in increasing need of energy generation which is directly connected to the increasing burning of fossil fuels ,resulting in growing pollution. This urgent need for an energy transition away from fossil fuels has led to the exploration of innovative technologies. Electrochemical CO2 reduction is one of the promising approaches that could evolve the energy landscape by producing chemicals and fuels from CO2, water, and renewable electricity.
Few Researchers from University of Cambridge have made a solar-driven reactor designed to convert captured CO2 also known as Thin Air and plastic waste into eco-friendly fuels. During experimentation, carbon dioxide was converted into syngas, a pivotal precursor for sustainable liquid fuels. Simultaneously, plastic bottles underwent conversion into glycolic acid, a commonly utilized component in the cosmetics industry. Wait, let me explain the whole process.
The Way to Make Fuel from CO2
So, this process also called as (MeOH) synthesis involves a
two-step procedure where the energy and molecules collected from a fossil fuel
or plastic, specifically methane (CH4), are transformed into MeOH (Methanol).
In the first step, steam-methane reforming is employed to convert CH4
into a mixture of H2, CO, and a small percentage of CO2.
This synthesis gas undergoes a subsequent conversion to MeOH in the second
step, operating at elevated temperatures (approximately 250°C) and pressures
(50–100 bar). While the direct electrochemical creation of MeOH from CO2
with high selectivities or current densities remains challenging, CO2
and water electrolysers offer the potential to replace the steam-methane
reforming step by producing CO and H2 from CO2 and H2O.
Essentially, the energy, carbon, and hydrogen content of CH4 are
substituted with solar energy, CO2, and H2O.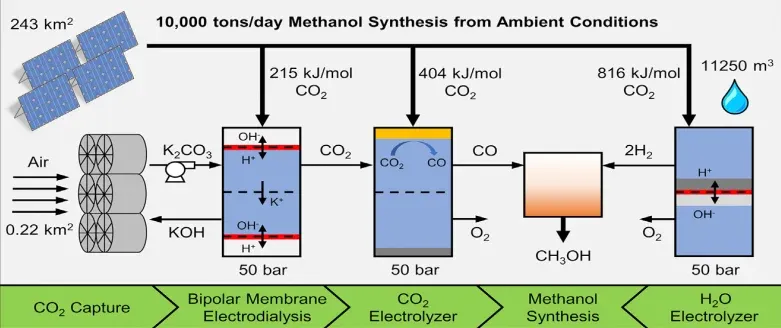
Credits to Reference No. 3
In the context of a purely solar-driven MeOH synthesis process, it is independent from the industrial inputs like CO2 from cement or steel plants, the CO2 and H2O molecules must originate from feedstocks initially in equilibrium with the environment. A feasible feedstock in this scenario is water sourced from the environment and CO2 from air at 400 ppm. To enable the operation of the CO2 electrolyser, the initially dilute atmospheric CO2 needs concentration. For the direct air capture (DAC) of CO2, potassium hydroxide (KOH) is employed as a capture solvent, converting ambient CO2 into carbonate upon contact. Subsequently, KOH and CO2 are recovered through electrically driven bipolar membrane electrodialysis (BPMED). The recovered KOH is recirculated to the capture unit, while CO2 remains in an aqueous electrolyte, pressurized to 50 bar in a KHCO2 electrolyte before being fed to the CO2 electrolyser. By using stoichiometric reaction and from various information collected ,the following equation is made :
For more information refer, reference no. 3. While the reaction scheme and specific technologies outlined here serve to create a realistic air-to-barrel scenario, various alternate processes are possible. It may take time before a final, preferred combination of technologies is established. Nonetheless, this exercise enables an analysis of energy requirements and physical scales for each sub-process in a 10,000 ton/day plant.
Future of This Method
The motivation behind conducting the above-mentioned analysis
is the exploration of how an air-to-barrel approach to CO2
conversion can offer an initial evaluation of practical operating conditions
and constraints for each step in the process. This holds particular
significance for CO2 electrolysers, which have yet to be thoroughly
examined within an integrated system, despite their anticipated role in the
energy transition. While the discussed case focuses on a specific set of
technologies, it underscores the necessity for CO2 capture technology to
seamlessly integrate with the CO2 conversion process. Additionally, if the CO2
conversion process does not yield a final product, it must integrate
effectively with downstream processing.
A more comprehensive analysis in the future will reveal more further
opportunities and upcoming expected limitations for solar fuel production
utilizing CO2 electrolysers as a conversion technology. This, in
turn, will facilitate meaningful comparisons with alternative technologies such
as reverse water-gas shift, direct CO2 to MeOH heterogeneous
catalysis, and solid-oxide CO2 electrolysis.
In our specific scenario, the constraints of MeOH synthesis and the requirements for BPMED dictate that CO2 electrolysis must be conducted at higher pressures than commonly reported in literature. Interestingly, the removal of CO2 as a gas from the recovered capture solvent through depressurization or regeneration demands additional energy compared to utilizing the saturated solution directly. Also, this case study establishes new boundary conditions essential for industrial CO2 electroreduction, merging both fundamental and practical research within realistic conditions.
Conclusion
As technology advances, opportunities will emerge to optimize system efficiencies by substituting and refining different components. Nevertheless, the essential inlet and outlet conditions are relatively fixed. For instance, the prospect of CO2 electroreduction in a gas-diffusion system instead of a pressurized aqueous system presents a notable possibility. Although removing gaseous CO2 from BPMED incurs a specific energy cost, potentially justifying the use of amine-based DAC over alkaline capture, the gains in efficiency during the CO2 reduction reaction may outweigh any additional energy requirements in the capture stage. However, unless the gas-diffusion system is pressurized, significant energy would be needed to compress CO and H2 for the synthesis step, good offsetting gains in overall efficiency. Additionally, replacing the BPMED step with a thermally driven release of CO2 from a capture solvent, utilizing waste heat, could enable the use of gas-diffusion layer configurations for CO2 reduction while reducing the renewable electrical energy requirements of the overall unit.
This detailed air-to-barrel case example is anticipated to assist the CO2 electroreduction community in evaluating relevant operating conditions crucial for scaling the technology practically. By considering these operating conditions and constraints, a new framework is provided to comprehend fundamental reaction phenomena and optimize catalyst, electrolyte, and reactor systems, serving as motivation to expedite the technology towards its ultimate goal. Envisaging the future energy and size requirements of CO2 electrolysers within a solar fuels process and comparing it to the current state of research allows for an assessment of when and how electrochemical CO2 reduction will contribute significantly to the imminent energy transition.
Reference : Finally, here I am giving credits to the websites and research paper which I got this information from especially the paper by Joule (reference no. 3). If you need any further information, please refer to the below articles and research papers :- https://bettersociety.net/fuel-from-air-cambridge.php
- https://www.science.org/doi/full/10.1126/science.365.6457.964
- Smith, W. A., Burdyny, T., Vermaas, D. A., & Geerlings, H. (2019). Pathways to industrial-scale fuel out of thin air from CO2 electrolysis. Joule, 3(8), 1822-1834.